The CEOs of Selexis SA and KBI Biopharma Answer Some Common Questions (An interview from BIO 2018)
Selexis CEO Igor Fisch, PhD, and KBI Biopharma President and CEO Tim Kelly, PhD, address the most common questions about being a part of JSR Life Sciences and their new collaboration, GENE to GMP in 9 Months
Q. JSR acquired KBI in 2015 and Selexis in 2017. Did you have a history of working together prior to the acquisition?
Igor: Yes. In fact, we engaged in our first partner project, a difficult-to-express bispecific already in in 2012. Since then, we have collaborated on more than 20 programs making a range of products from monoclonal antibodies (mAbs) to bispecifics and fusion proteins. I believe KBI has performed GMP manufacturing with more than 10 of our cell lines.
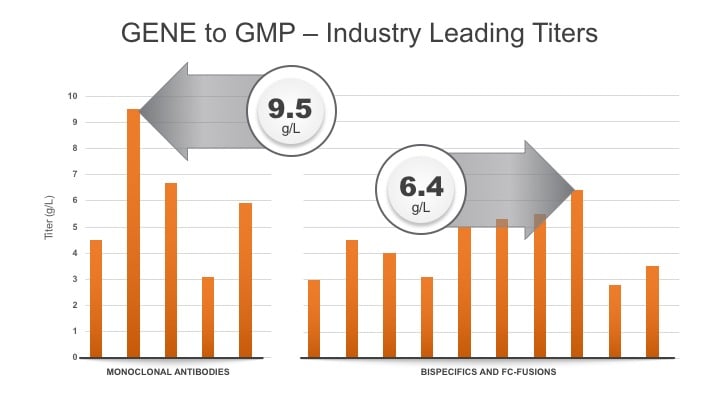
Tim: That is correct. I also want to point out that, as a result these 20 plus projects, we believe we offer industry-leading titers for a broad range of product types. For monoclonal antibodies, we have achieved titers as high as 9.5 g/L. For bispecifics and fc-fusions, we average >4.3 g/L and 6.4 g/L respectively at bioreactor harvest (see graph below).
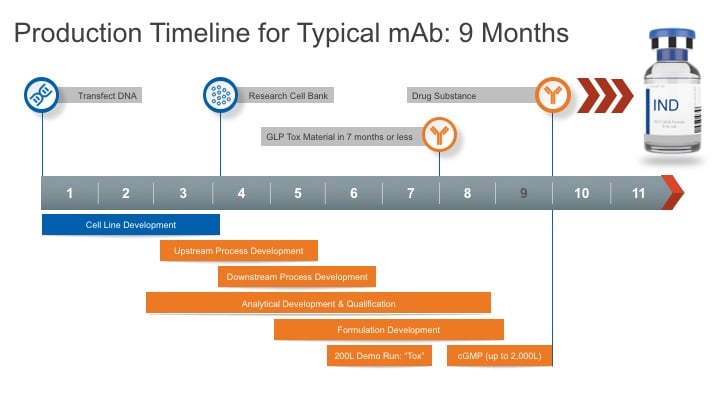
Q. You recently launched Gene to GMP in 9 Months. What does Gene to GMP mean?
Igor: Our Gene to GMP service offering brings together the collective expertise of both KBI and Selexis to deliver a seamless process for fast development of protein therapeutics without sacrificing quality or analytics. For conventional mAbs and more readily-expressible proteins, a partner provides us with their gene sequence and we advance the development process all the way to clinical grade API, active pharmaceutical ingredient, in nine months. The Selexis team brings more than 17 years of cell line development technology and experience to this collaboration. With our accelerated research cell bank program, we can generate stable and high expressing RBCs in 14 weeks.
Tim: Dovetailing off of what Igor said about no sacrifice in quality or analytics, at KBI, we are driven by data. We believe that reliable high quality manufacturing can only be achieved with a thorough understanding of the protein at the biochemical and structural level. As a result, we have built deep analytics capabilities in-house that support all aspects of our process development and manufacturing services. In our experience, detailed protein characterization improves decision-making and drives speed in development and manufacturing. Our capabilities perfectly complement Selexis’ expertise, enabling us to work together to bring this important offering to our partners.
Q. Not all proteins are readily expressed or secreted from CHO cells. How do your two organizations work together on difficult-to-express proteins?
Igor: The pressures on CHO cells to express complex and difficult-to-express proteins led us to develop modules for our SUREtechnology Platform that address a range of issues. For example, we developed the SURE CHO-Mplus Libraries to address translation/secretion bottlenecks, SUREvariant Screening for lead candidate selection and, last but not least, SUREscan and SUREsignature for quality assessment and barcoding of the clonal production cell line using next generation sequencing methods.
Tim: Difficult-to-express proteins are often also difficult to purify, and we frequently cannot rely on conventional high-yield affinity chromatography steps such as Protein A. KBI has built exceptionally strong process development capabilities to solve the challenges of the new generation of non-mAb products and successfully deliver these innovative drugs to patients.
Q. What are some the most difficult or complicated projects you have worked on together?
Tim: As we have both mentioned, many of our clients are developing bispecifics and other novel constructs. These products frequently pose significant challenges for both cell line expression and production. Selexis’ track record of achieving industry-best titers for such products is outstanding, and KBI supports this with the high-resolution analytics necessary to ensure the selected clone meets product quality requirements, such as high heterodimer ratio for bispecifics. Together, KBI and Selexis have an exceptional track record of delivering the highest quality process and product for bispecifics under accelerated development timelines.
Q. What do you see in the future for biologics generation and manufacturing?
Igor: Next-generation protein biologics are increasingly complex to manufacture. We are already seeing this with molecules like bispecific t-cell engagers mAbs and immunocytokines that recruit the immune system for cancer control. These complex molecules will push the secretory limits of the current CHO cells. Based on the extensive genomic, transcriptomic metabolomic analysis of our proprietary CHO-K1 cell line, we are continuing to improve our CHO cell line and offer modular solutions to be able to accommodate these newer, non-natural proteins.
Tim: I agree with Igor. We will continue to see increasingly complex molecules that will pose unique challenges for process development, scale-up, and predictable cGMP production. KBI is uniquely qualified to overcome these challenges because of our exceptionally strong and deep analytical and process development capabilities. Today, KBI and Selexis are on the leading edge of novel biologics development and together we are building the foundation for the even more challenging therapeutics of tomorrow.
For more information about GENE to GMP in 9 Months, please visit www.GtoG.bio.
For more information about KBI’s analytic and manufacturing capabilities, visit www.kbibiopharma.com.



